-
LIU Gang Title:Professor Email: gangliu@imr.ac.cn Tel. : +86-24-23971088 FAX: +86-24-23971682 Division: Shenyang National Laboratory for Materials Science Address: Advanced Carbon Division, Institute of Metal Research Chinese Academy of Sciences (IMR CAS), 72 Wenhua Road,Shenyang,China, 110016
Experience: |
|
Education 2009 Ph.D Materials Science, Institute of Metal Research, Chinese Academy of Sciences (CAS), China Work experience 2014.10-present Professor, Institute of Metal Research, CAS |
Research Interest: |
|
Designing photocatalytic materials for solar fuels |
Research Achievement: |
|
Photocatalysis efficiency is intrinsically determined by three aspects of photocatalysts, namely light absorption, separation and transfer of photogenerated charge carriers, and surface catalysis of materials ( Achieving wide-spectrum absorption of solar light The aim is to increase visible light absorption of wide-bandgap semiconductors by introducing heteroatoms or defects so that solar light can be fully harvested. Our major concern is how to realize the band-to-band redshift of light absorption edge by controlling the spatial distribution of dopants. Meanwhile, unknown visible light responsive photocatalysts consisting of earth-abundant elements will be explored.
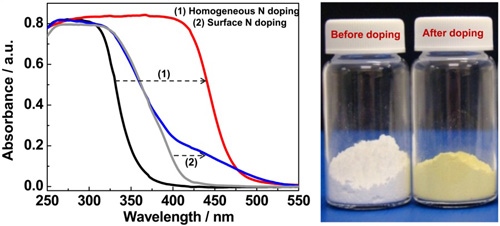
Figure 1 Homogeneous N doping in Cs0.68Ti1.83O4. The left panel: UV-visible absorption spectra of (1) homogeneous N doped Cs0.68Ti1.83O4 and (2) surface N doped TiO2. The right panel: optical photograph of Cs0.68Ti1.83O4 samples before and after homogeneous N doping. (Band-to-band visible-light photon excitation and photoactivity induced by homogeneous nitrogen doping in layered titanates, Chem Mater 2009, 21, 1266-1274)
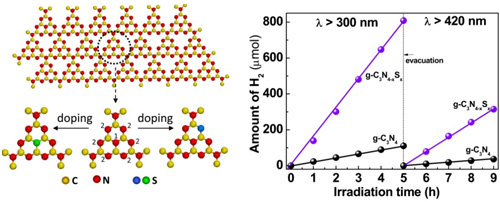
Figure 2 Homogeneous S doping in g-C3N4. The left panel: schematic of two lattice N sites for substitutional S in perfect graphitic carbon nitride. The right panel: a typical time course of hydrogen evolution from water containing 10 vol% triethanolamine scavenger by Pt-deposited g-C3N4 (a) and g-C3N4-xSx (b) under λ > 300 and 420 nm. (Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4, J Am Chem Soc 2010, 132, 11642-11648)
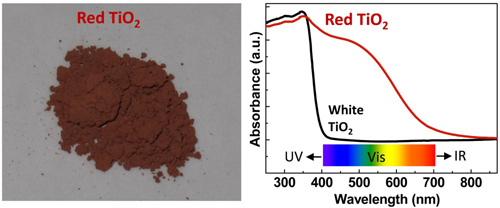
Figure 3 A red anatase TiO2 with a gradient B/N doping. The left panel: optical photograph of the prepared red TiO2 sample. The right panel: UV-visible absorption of the white TiO2 and red TiO2. (A red anatase TiO2 photocatalyst for solar energy conversion, Energy Environ Sci 2012, 5, 9603-9610)
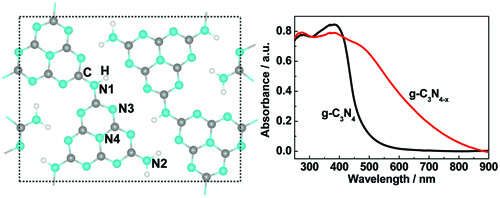
Figure 4 Homogeneous modification with nitrogen vacancies in g-C3N4. The left panel: schematic of the two dimensional sheets of pristine g-C3N4 (melon). The right panel: UV-visible absorption spectra of g-C3N4 and g-C3N4-x (obtained by reducing g-C3N4 in a hydrogen atmosphere). (Increasing the visible light absorption of graphitic carbon nitride (melon) photocatalysts by homogeneous self-modification with nitrogen vacancies, Adv Mater 2014, 26, 8046)
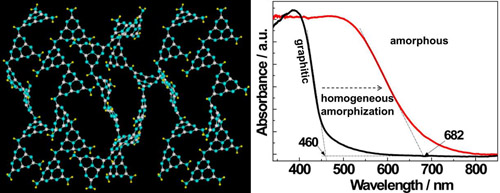
Figure 5 Homogeneous amorphization of g-C3N4. The left panel: schematic of the two dimensional sheets of disordered pristine g-C3N4. The right panel: UV-visible absorption spectra of g-C3N4 and amorphous C3N4 (obtained by heating g-C3N4 in an argon atmosphere). (An amorphous carbon nitride photocatalyst with greatly extended visible-light-responsive range for photocatalytic hydrogen generation, Adv. Mater., 2015, 27, 4572)
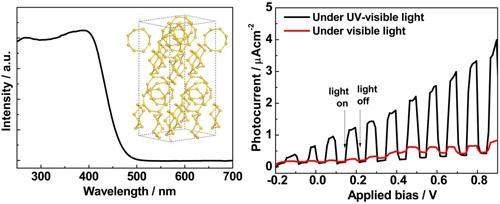
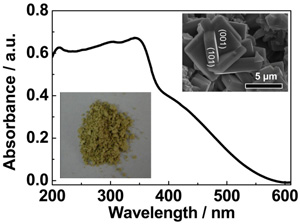
Figure 6 α-S photocatalyst. The left panel: UV-visible absorption spectrum of α-sulfur. The inset is a photograph of the α-S crystal powder. The right panel: Applied potential bias dependence of the photocurrent generated by the photoanode of α-S crystals under UV-visible and visible light irradiation. (α-sulfur crystals as a visible light active photocatalyst, J Am Chem Soc 2012, 134, 9070-9073)
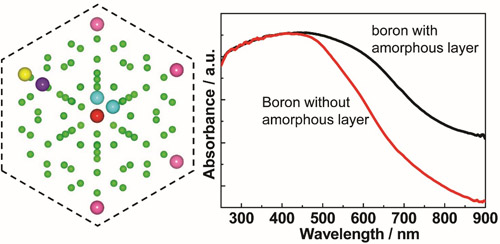
Figure 7 β-boron photocatalyst. The left panel: schematic of atomic structure of β-boron. The right panel: UV-visible absorption spectra of boron powder with and without surface amorphous layer (Visible-light-responsive β-rhombohedral boron photocatalysts, Angew Chem Int Ed 2013, 52, 6242-6245) Promoted separation and transfer of photogenerated charge carriers To promote the separation and trasnfer of photogenerated electrons and holes in semiconductor based photocatalysts, two strategies are used. One is shortening the diffusion length of the charge carriers from the bulk to surface by decreasing the size along one or two dimensions of photocatalysts to nanometers so that the recombination of photoexcited charge carriers will be greatly lowered. The other is to enable spatial distribution of photogenerated electrons and holes between two components or regions of one component in heterostructured photocatalysts.
Figure 8 g-C3N4 nanosheets. SEM images of bulk g-C3N4 and g-C3N4 nanosheets (Graphene-like carbon nitride nanosheets for improved photocatalytic activities, Adv Funct Mater 2012, 22, 4763-4770)
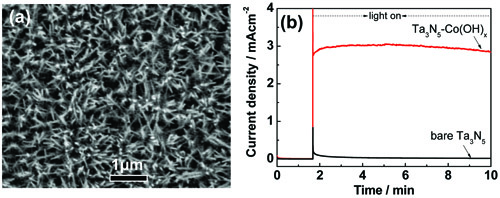
Figure 9 Photoanode of Ta3N5 nanorod arrays. SEM image of Ta3N5 nanorod arrays supported on Ta substrate and photoelectrochemical water oxidation activity of Co(OH)x modified Ta3N5 (Template-free synthesis of Ta3N5 nanorod arrays for efficient photoelectrochemical water splitting, Chem Commun 2013, 49, 3019-3021)
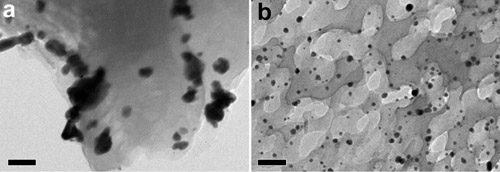
Figure 10 TEM images of (a) pristine g-C3N4 and (b) porous g-C3N4 photocatalysts after loading Au particles (black particles) via a photodeposition method. The spatial distribution of Au particles on photocatalysts shows the abundance of reductive sites. Scale bars are 50 nm. (Selective breaking of hydrogen bonds of layered carbon nitride towards greatly enhanced visible light photocatalysis, Adv. Mater., 2016, 28, 6471–6477)
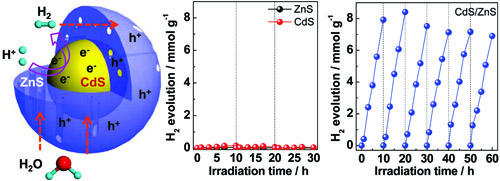
Figure 11 CdS/ZnS core-shell particles. The left panel: schematic of CdS-mesoporous ZnS core-shell particles with the separation of charge carriers. The middle and right panels: photocatalytic hydrogen generation with ZnS, CdS, and the core-shell particles from the aqueous solution of Na2S/Na2SO3 under visible light. (CdS-mesoporous ZnS core-shell particles for efficient and stable photocatalytic hydrogen evolution under visible light, Energy Environ Sci 2014, 7, 1895–1901)
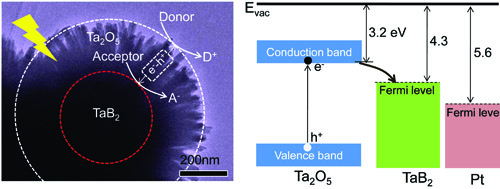
Figure 12 TaB2/Ta2O5 core/shell particles. The left panel: schematic of a TEM image of TaB2/Ta2O5 core/shell particles with a function of promoting the separation of photoexcited electrons and holes. The right panel: band alignment of Ta2O5 referring to Fermi level of TaB2 and Pt as co-catalyst. (Constructing metallic/semiconducting TaB2/Ta2O5 core/shell heterostructure for photocatalytic hydrogen evolution, Adv Energy Mater 2014, 4, 1400057)
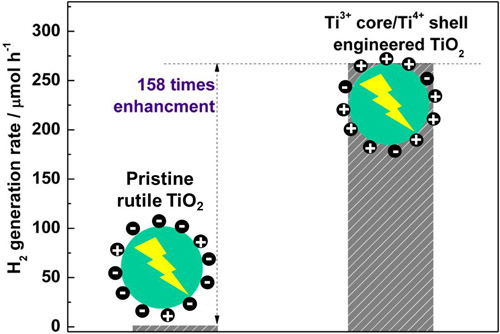
Figure 13 Comparison of photocatalytic hydrogen generation from mixture of water/methanol with pristine rutile TiO2 and Ti3+/Ti4+ core/shell rutile TiO2 particles after loading 1 wt% Pt co-catalyst. (Enhanced photocatalytic H2 production in core-shell engineered rutile TiO2, Adv. Mate., 2016, 28, 5850-5856) Faceting mediated catalysis The aim is to modulate surface atomic structures of photocatalysts by controlling the selective exposure of different crystal facets during the growth of crystals. These faceted photocatalysts provide an excellent platform to reveal surface structure dependent photocatalytic activity.
Figure 14 N doped anatase TiO2 crystal with dominant {001} facets. UV-visible absorption spectrum of nitrogen doped anatase TiO2 crystals with dominant {001}. The insets are optical photograph and SEM image of nitrogen doped anatase TiO2 crystals with dominant {001}. (Visible light responsive nitrogen doped anatase TiO2 sheets with dominant {001} facets derived from TiN, J Am Chem Soc 2009, 131, 12868-12869)
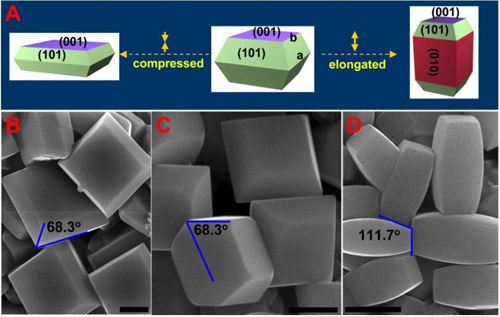
Figure 15 Anatase TiO2 crystals with a predominance of low index facets. Schematic (A) and SEM images (B-D) of anatase TiO2 single crystals with different percentages of {001}, {101}, and {010} facets. (On the true photoreactivity order of {001}, {010} and {101} facets of anatase TiO2 crystals, Angew Chem Int Ed 2011, 50, 2133-2137)
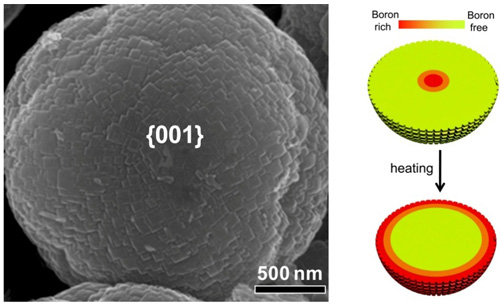
Figure 16 {001} dominated Anatase TiO2 microspheres with tunable spatial distribution of boron. The left panel: SEM images of anatase TiO2 microsphere with nearly 100% {001} surface. The right panel: schematic of boron distribution in the microsphere before and after heating. (Heteroatom-modulated switching of photocatalytic hydrogen and oxygen evolution preferences of anatase TiO2 microspheres, Adv Funct Mater 2012, 22, 3233–3238)
Figure 17 Crystal facet dependent interfacial electric conductivity in faceted anatase TiO2 crystal. I-V curves along different crystallography orientations were measured by contacting one TiO2 particle with two tungsten probes in SEM microscope. (Greatly enhanced electronic conduction and lithium storage of faceted TiO2 crystals supported on metallic substrates by tuning crystallographic orientation of TiO2, Adv. Mater., 2015, 27 3507–3512)
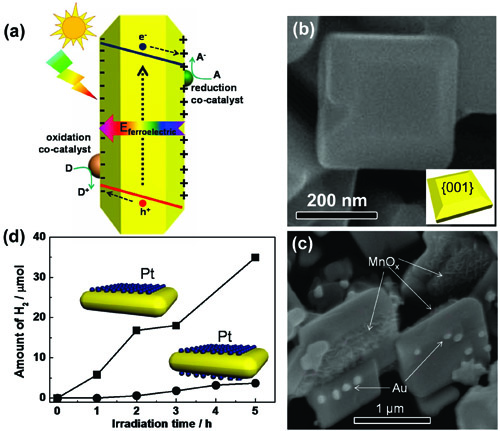
Figure 18 Ferroelectric field assisted selective deposition of co-catalysts on different sides of facet. (a) Schematic of single-domain & single crystalline ferroelectric material with in-built electric field; (b) SEM image of PbTiO3 nanoplates with dominant {001} facets; (c) SEM image of PbTiO3 nanoplates with a selective depositionof Au and MnOx on different sides; (d) Comparison of photocatalytic hydrogen generation between the PbTiO3 with the selective deposition of reducing co-catalyst Pt and the PbTiO3 with the nonselective deposition of reducing co-catalyst Pt. (Selective Deposition of Redox Co-catalysts to Improve the Photocatalytic Activity of Single-Domain Ferroelectric PbTiO3 Nanoplates, Chemical Communications 2014, 50, 10416 -10419) |
Service to the International Professional Societies: |
Honors: |
Publication: |
|
1. Y. Y. Kang, Y. Q. Yang, L. C. Yin, X. D. Kang, L. Z. Wang, G. Liu,* H. M. Cheng, Selective breaking of hydrogen bonds of layered carbon nitride towards greatly enhanced visible light photocatalysis, Advanced Materials, 28(30), 6471–6477, (2016). 2. Y. Q. Yang, G. Liu,* J. TS Irvine, H. M. Cheng,* Enhanced photocatalytic H2 production in core-shell engineered rutile TiO2, Advanced Materials, 28(28), 5850-5856, (2016). 3. G. Zhang, G. Liu,* L. Z. Wang,* J. TS Irvine,* Inorganic perovskite photocatalysts for solar energy utilization, Chemical Society Reviews, 45 (21), 5951-5984 (2016). 4. Y. Y. Kang, Y. Q. Yang, L. C. Yin, X. D. Kang, G. Liu,* H. M. Cheng, An amorphous carbon nitride photocatalyst with greatly extended visible-light-responsive range for photocatalytic hydrogen generation, Advanced Materials, 27(31), 4572–4577, (2015). 5. G. Liu, L. C. Yin, J. Pan, F. Li, L. Wen, C. Zhen, H. M. Cheng*, Greatly enhanced electronic conduction and lithium storage of faceted TiO2 crystals supported on metallic substrates by tuning crystallographic orientation of TiO2, Advanced Materials, 27(23), 3507–3512, (2015). 6. P. Niu, L. C. Yin, Y. Q. Yang, G. Liu,* H. M. Cheng, Increasing the visible light absorption of graphitic carbon nitride (melon) photocatalysts by homogeneous self-modification with nitrogen vacancies, Advanced Materials 26 (47), 8046–8052, (2014). 7. Y. Q. Yang, C. H. Sun, L. Z. Wang, Z. Liu, G. Liu,* X. L. Ma, H. M. Cheng, Constructing metallic/semiconducting TaB2/Ta2O5 core/shell heterostructure for photocatalytic hydrogen evolution, Advanced Energy Materials 4 (12), 1400057, (2014). 8. G. Liu, H. G. Yang, J. Pan, Y. Q. Yang, G. Q. Lu,* H. M. Cheng,* Titanium dioxide crystals with tailored facets, Chemical Reviews 114 (19), 9559?9612, (2014). 9. Y. P. Xie, Z. B. Yu, G. Liu,* X. L. Ma, H.-M. Cheng,* CdS-mesoporous ZnS core-shell particles for efficient and stable photocatalytic hydrogen evolution under visible light, Energy & Environmental Science 7 (6), 1895–1901, (2014). 10. G. Liu, L. C. Yin, P. Niu, W. Jiao, H. M. Cheng,* Visible-light-responsive β-rhombohedral boron photocatalysts, Angewandte Chemie International Edition 52 (24), 6242-6245, (2013). 11. P. Niu, L. L. Zhang, G. Liu*, H. M. Cheng, Graphene-like carbon nitride nanosheets for improved photocatalytic activities, Advanced Functional Materials 22 (22), 4763-4770, (2012). 12. G. Liu, L.-C. Yin, J. Q. Wang, P. Niu, C. Zhen, Y. P. Xie, H.-M. Cheng,* A red anatase TiO2 photocatalyst for solar energy conversion, Energy & Environmental Science 5 (11), 9603-9610, (2012). 13. G. Liu, P. Niu, L. C. Yin, H. M. Cheng*, α-sulfur crystals as a visible light active photocatalyst, Journal of the American Chemical Society, 134 (22), 9070-9073, (2012). 14. G. Liu, J. Pan, L. C. Yin, J. TS Irvine, F. Li, J. Tan, P. Wormald, H.-M. Cheng*, Heteroatom-modulated switching of photocatalytic hydrogen and oxygen evolution preferences of anatase TiO2 microspheres, Advanced Functional Materials 22 (15), 3233–3238, (2012). 15. J. Pan, G. Liu*, G. Q. (Max) Lu, H. M. Cheng*, On the true photoreactivity order of {001}, {010} and {101} facets of anatase TiO2 crystals, Angewandte Chemie International Edition 50 (9), 2133-2137, (2011). |
Homepage: |